Media
HKUMed uncovers the mechanism behind exercise-induced bone strengthening, paving the way for novel osteoporosis treatment
29 Dec 2025

HKUMed uncovered the mechanism behind bone strength, paving the way for novel osteoporosis treatment. The research was led by Professor Xu Aimin (second right) and Dr Wang Baile (second left, front row) from the Department of Medicine, the School of Clinical Medicine, HKUMed.

The research team identified clear intervention targets for treating osteoporosis, aiding in the development of drugs that simulate the benefits of exercise. This provides the potential for targeted treatments for vulnerable groups, such as those who are bedridden or have limited mobility, offering benefits comparable to those of physical activity.
A research team from the Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine at the University of Hong Kong (HKUMed) has uncovered a key biological mechanism that explains how exercise maintain strong bones, paving the way for novel treatments for osteoporosis and bone loss in people who are unable to engage. By identifying a protein that acts as the body’s ‘exercise sensor’, the research has opened the door to the development of drugs that mimic the effects of physical activity, offering hope for vulnerable groups, such as the elderly, bedridden patients and those with chronic illnesses who face a high risk of fractures. The research findings were published in the journal Signal Transduction and Targeted Therapy [link to the publication].
‘Osteoporosis and age-related bone loss affect millions worldwide, often leaving elderly and bedridden patients vulnerable to fractures and loss of independence,’ said Professor Xu Aimin, Director of the State Key Laboratory of Pharmaceutical Biotechnology and Chair Professor in the Department of Medicine, School of Clinical Medicine, HKUMed, who led the study. ‘Current treatments rely heavily on physical activity, which many patients simply cannot perform. We need to understand how our bones get stronger when we move or exercise before we can find a way to replicate the benefits of exercise at the molecular level. This study is a critical step towards that goal.’
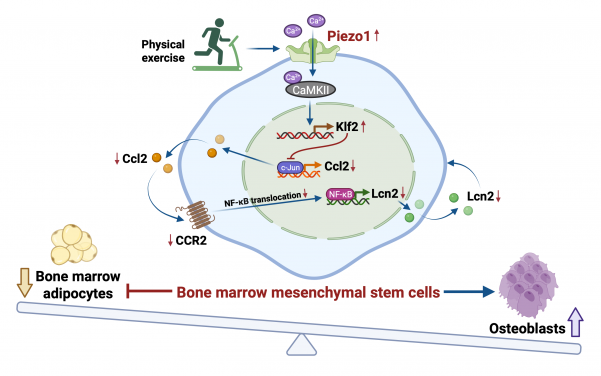
Activating the bone’s ‘exercise sensor’ to reduce fat and build new bone
According to the World Health Organization, approximately 1 in 3 women and 1 in 5 men over the age of 50 suffer a fracture due to weak bones. In Hong Kong, the problem is significant as the population ages, with 45% of women and 13% of men aged 65 and above affected by osteoporosis. Osteoporosis-related fractures cause significant pain and disability, severely impacting quality of life and independence, while placing a heavy healthcare and economic burden on the society.
Ageing involves the natural weakening of bones, which become less dense and more porous. Mesenchymal stem cells in the bone marrow have the potential to develop into either fat cells or bone tissue. These stem cells are highly sensitive to external factors, like exercise and pressure. During the ageing process, they tend to differentiate into fat cells. As fat cells continue to accumulate in the bone marrow, it reduces the space available for healthy bone tissue, further compromising bone strength and creating a cycle of bone deterioration that is difficult to reverse with current treatments.
Using mouse models and human stem cells, the researchers identified a special ‘switch’, called Piezo1, which is a protein on the surface of mesenchymal stem cells in the bone marrow. This switch acts like an exercise sensor, detecting mechanical signals from physical activity that helps keep bones strong and preventing them from becoming frail as we age. In the mice model, when the Piezo1 protein was activated by physical activity, it reduced fat accumulation in the bone marrow and encouraged the formation of new bone. Conversely, when this protein is missing, it triggers signals that cause stem cells to accumulate fat, leading to increased bone loss. The absence of Piezo1 also promotes the release of certain pro-inflammatory signals (Ccl2 and lipocalin-2), making stem cells more likely to convert into fat cells, which hinders bone formation. Blocking these signals can help restore bone health.
Mimicking exercise for individuals with limited mobility
’We have essentially decoded how the body converts movement into stronger bones,’ said Professor Xu Aimin. ‘We have identified the molecular exercise sensor, Piezo1, and the signalling pathways it controls. This gives us a clear target for intervention. By activating the Piezo1 pathway, we can mimic the benefits of exercise, effectively tricking the body into thinking it is exercising, even in the absence of movement.’
Dr Wang Baile, Research Assistant Professor from the same department, who co-led the research, added, ‘This discovery is especially meaningful for older individuals and patients who cannot exercise due to frailty, injury or chronic illness. Our findings open the door to developing “exercise mimetics” — drugs that chemically activate the Piezo1 pathway to help maintain bone mass and support independence.’
‘This offers a promising strategy beyond traditional physical therapy,’ remarked Professor Eric Honoré, Team Leader at the Institute of Molecular and Cellular Pharmacology, French National Centre for Scientific Research, who co-led the research. ‘In the future, we could potentially provide the biological benefits of exercise through targeted treatments, thereby slowing bone loss in vulnerable groups such as the bedridden patients or those with limited mobility, and substantially reducing their risk of fractures.’
The research team is now working to translate these findings into clinical applications, with the goal of developing new treatments to preserve bone health and improve the quality of life for an ageing population and those confined to bed.
About the research team
The collaborative study was co-led by Professor Xu Aimin, Rosie T T Young Professor in Endocrinology and Metabolism, Chair Professor and Director; and Dr Wang Baile, Research Assistant Professor, the State Key Laboratory of Pharmaceutical Biotechnology, Department of Medicine, HKUMed; in collaboration with Professor Eric Honoré from Institute of Molecular and Cellular Pharmacology, French National Centre for Scientific Research (CNRS), Université Côte d’Azur (UniCA), and the French National Institute of Health and Medical Research (Inserm), who is also a Visiting Professor in the Department of Pharmacology and Pharmacy, HKUMed.
Acknowledgments
This research was supported by the Areas of Excellence Scheme, and the General Research Fund of the Research Grants Council; the Health and Medical Research Fund under the Health Bureau, the Government of the Hong Kong Special Administrative Region of the People’s Republic of China; National Key R&D Program of China; the National Natural Science Foundation of China; the Human Frontier Science Program; the French National Research Agency; Fondation de France; Fondation pour la Recherche Médicale; and the Macau Science and Technology Development Fund.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).